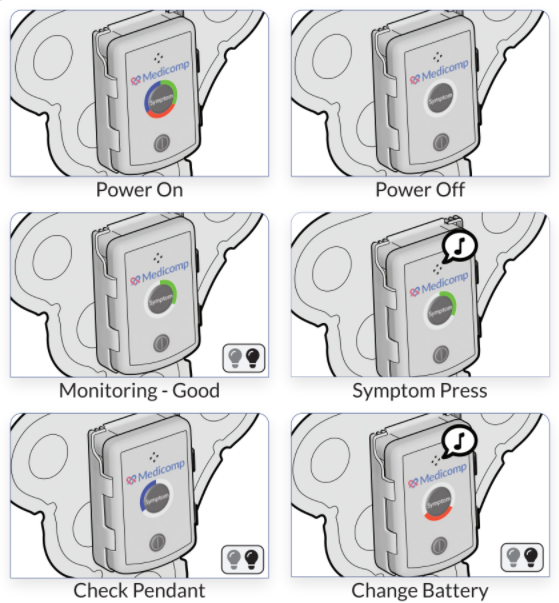
ReactDx has a long-standing history of supplying researchers with portable ECG monitors for over 20 clinical trials worldwide. Our close ties with improving healthcare has led to a better understanding of clinical trials and their varied methodologies. Below is a short synopsis of different clinical trials, along with significant information on each phase of the trial.
When researchers form a hypothesis concerning patient healthcare, a clinical trial is necessary before a conclusion can be reached. Clinical trials are categorized as either observational or interventional. An observational clinical trial monitors patient health over a set time frame, and no drugs or treatments are given. These trials give a better understanding of disease processes and how they advance. Interventional clinical trials, on the other hand, use medications, therapy, and/or experimental treatment to assess safety and effectiveness when utilized by a group of individuals.
These two broad categories can be broken into classifications.
- Diagnostic trials: Explore better methods of identifying a specific disorder.
- Epidemiological trials: Identify patterns, causes, and control of disorders in populations.
- Genetic trials: Improve prediction rates of disorders through better understanding and identification of the interrelationship of genes and disease. Genetic trials may lead to tailor-designed treatments based on an individual’s genetics.
- Prevention trials: Emphasize preventing disorders from developing or returning through the advent of medication, vitamins and minerals, vaccines, lifestyle alterations, et cetera.
- Quality-of-life trials: Explore improved comfort and quality of life for patients with end-of-stage illnesses.
- Screening trials: Find the best detection method for specific disorders or conditions. Emphasis is generally on the earlier stages of disease.
- Treatment trials: Focus on alleviating symptoms or slowing the progression of disease through medication combinations, psychotherapy, technology, surgery, et cetera.
The U.S. Food and Drug Administration (FDA) requires all these trials to follow specific phases.
- Phase I trials establish if a potential drug or treatment is safe for humans. These trials generally involve a small sampling of patients — from 10 to 80 on average — to assess safety, side effects, method of delivery, and dosage levels.
- Phase II trials evaluate safety and effectiveness of a potential drug or treatment on a larger patient sample — generally 100 to 300 participants.
- Phase III trials confirm a potential drug or treatment’s effectiveness while monitoring side effects. The drug or treatment is compared with a well-established drug or treatment. A much larger contingency is employed, generally from 1,000 to 3,000 individuals. Phase III trials are lengthy compared with the initial 2 phases.
- Phase IV trials are a post-marketing phase occurring after the drug or treatment has received FDA approval. This trial phase evaluates the drug or treatment’s potential for additional uses beyond the scope of the original intent and accounts for any long-term side effects.
ReactDx’s participation in clinical trials has aided researchers tremendously. The advanced technology of ReactDx’s line of ECG monitors track rate, rhythm, morphology, and P-wave analysis. Contact ReactDx today at 800-23-HEART to speak with one of our knowledgeable representatives.