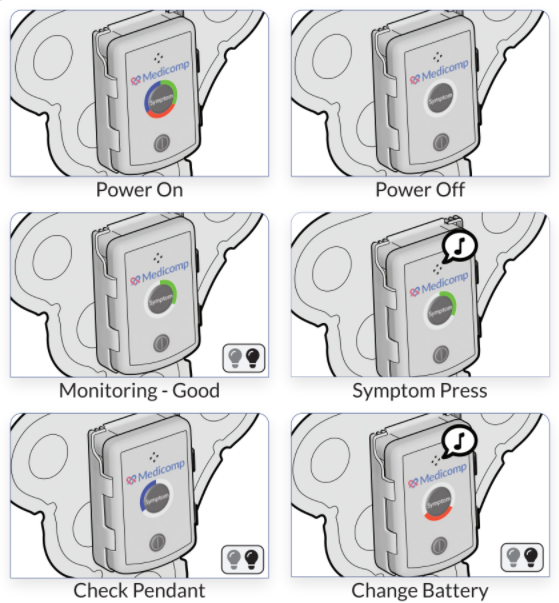
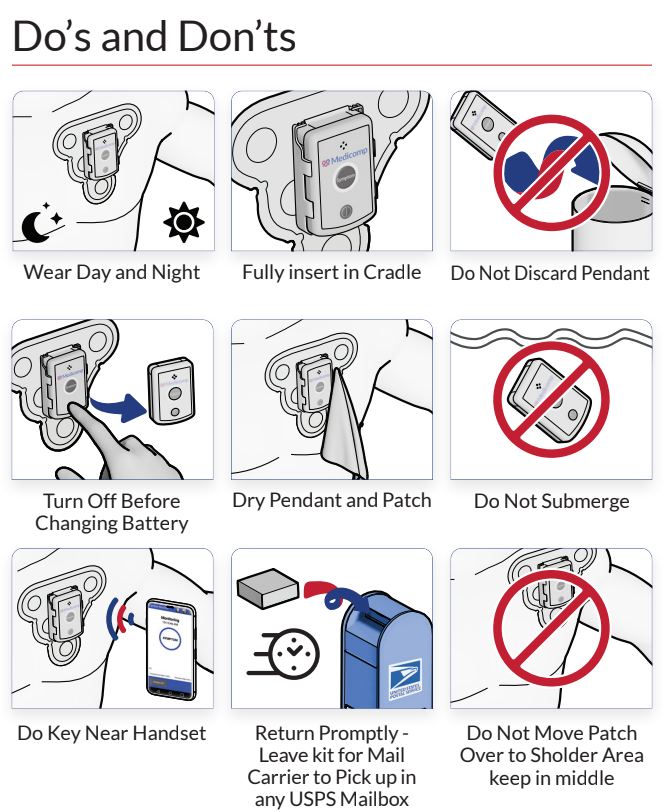
Duke University and the Food and Drug Administration (FDA) jointly formed the Clinical Trial Transformation Initiative (CTTI), which released a report stating that all clinical trials should utilize mobile technology. ReactDx has a long-standing role as a technology leader in clinical trials with their remote cardiac monitors. The following information from the CTTI’s report contains information on how wearable technology can stimulate clinical trial participants.
- Because a large portion of the population is familiar with wearable technology to capture heart rate, exercise level, step count, blood glucose level, amount of sleep, calories consumed and burned, and more, participation and compliance increase.
- Pundits believe trials may be thwarted by technology since participants send reports from home rather than driving to a clinic for testing. The FDA is concerned with the quality, reliability, and integrity of data, and that clinical trial supporters must find appropriate technology beforehand. CTTI countered that if the FDA approved of the devices utilized in each clinical trial, the trials could very possibly include smartphones, wearable technology, ingestible or implantable technology, or any information shared via laptop or tablet.
- Because most participants currently own much of the wearable technology used in clinical trials, initial costs would be low.
- Rather than compiling reports weekly, monthly, or quarterly, information is gathered immediately and can be compared from one participant to the next in real time. Participants become more engaged in clinical trials when they are able to see how their results affect the trial.
- Feasibility testing before clinical trials begin can evaluate and determine the ultimate technology for each trial before full-scale implementation.
CTTI stresses this approach for all clinical trials is imminent. ReactDx’s remote cardiac monitors have headlined several clinical trials with stellar results. Learn how your patients can benefit from any of ReactDx’s wearable heart rate monitors to safeguard and track their heart health by calling 800-23-HEART.