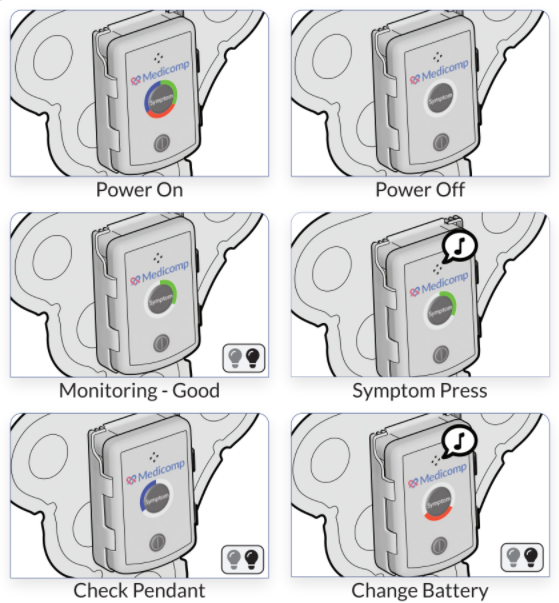
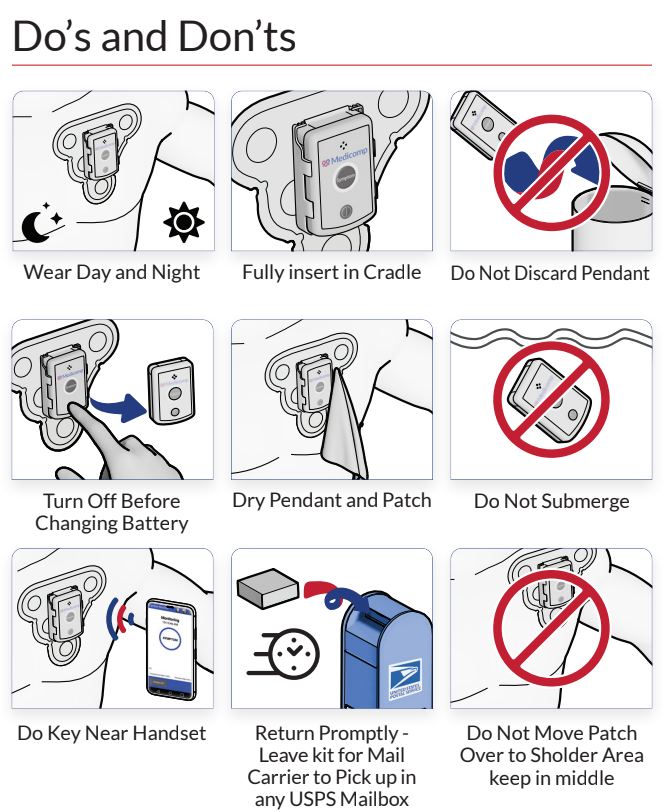
Peripheral artery disease (PAD) affects the vascular system, specifically in the arms, legs, and head. Most instances of plaque buildup, which consists of fats and cholesterol deposited along the arterial walls and are directly associated with PAD, occur in the lower extremities. The calcification of plaque creates an even more dangerous situation since the brittle plaque buildup can break free and block blood flow.
The sound wave technology designed for the Lithoplasty System reduces plaque size considerably so it can be passed harmlessly out of the body while preserving the friable epithelial tissue lining the blood vessel. To maintain arterial flow within the area where the plaque resided, balloon catheterization safely expands the vessel to allow normal blood flow.
Shockwave Medical focused on PAD patients with calcified plaques, a population chronically considered difficult to treat. This new technology minimizes vascular injuries while relieving arterial pressure. Future procedures utilizing the Lithoplasty System will no longer require stents, dissection of vessels, or other interventions that often lead to complications.
As research and technology determine new and innovative procedures to diagnose and treat cardiovascular disease such as peripheral artery disease, patients can live longer, healthier lives. ReactDx is dedicated to patient care. Our line of patch monitoring systems can evaluate whether patients who have recently had this or any other cardiovascular procedure are experiencing cardiac anomalies associated with heart disease. Give ReactDx a call at 800-234-3278 (800-23-HEART) and speak with one of our trained representatives. Our blogs discuss the latest technology for patients and physicians, diet, exercise, and heart-healthy tips.